TAVR Procedures Can Cause
Brain Injury
Risks in detail
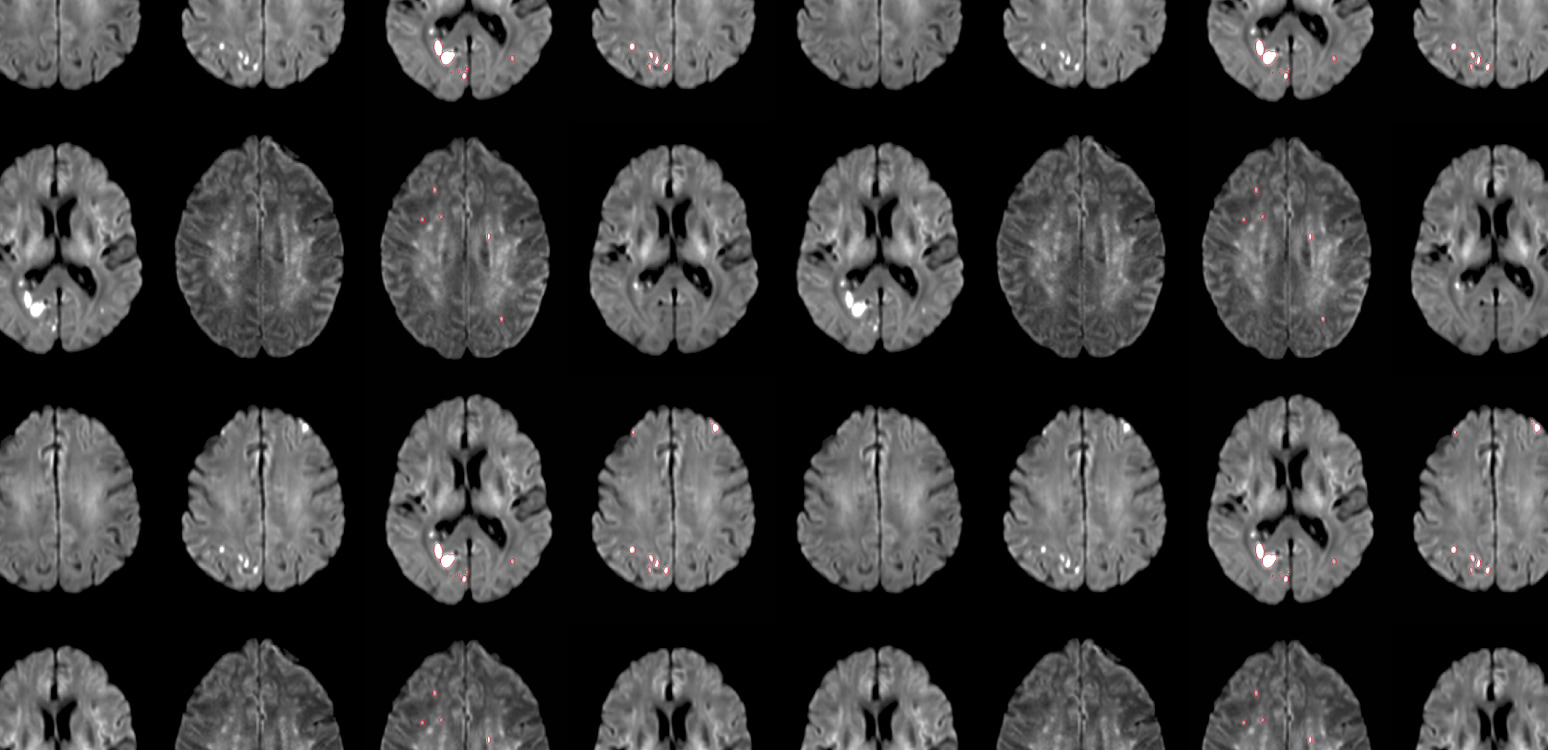
An acute lesion identified by diffusion weighted magnetic resonance imaging (DW-MRI) is generally a reliable signature of infarct and represents mostly permanent brain damage – sustained reversal is infrequent.3 These lesions are usually equally distributed in all major cerebral vascular territories except that of the Anterior Cerebral Artery.4,5
- Middle Cerebral Territory: 38%
- Posterior Cerebral Territory: 33%
- Cerebellar Brainstem: 27%
- Anterior Cerebral Territory: 2%
Silent brain infarcts, identified by DW-MRI, occur in the large majority of TAVR procedures with various associated risks.6
The number, size, and total volume of acute brain infarctions identified by DW-MRI are each associated with clinical ischemic strokes, disabling strokes, and worse stroke recovery in patients undergoing TAVR, and have value as surrogate outcomes in stroke prevention trials.7
Large population-based studies demonstrate associations between MRI lesions and clinical stroke, mortality, and cognitive decline.10
Infarcts are associated with brain volume reduction, but importantly, also with detectably lower cognition.1 Studies show that cognitive decline relates directly to loss of brain substance with progression of lesion burden.12
Cerebral infarcts have been linked to neurocognitive decline by affecting global memory function, dementia and hippocampal function and structure.13
Moreover, additional new infarcts are associated with more cognitive decline and an even higher risk for dementia than just having prevalent infarcts.14
Cerebral embolic protection (CEP) has become even more important as the TAVR patient profile is shifting to younger and lower risk patients.15 12.2% of patients with aortic stenosis (AS) aged <65 years receiving aortic valve replacement were treated with TAVR, and 45% of patients in the US receiving TAVR were younger than 65 years of age.16
First-Generation CPS has Equivocal Clinical Results
Currently, there is only one cerebral protection system (CPS) commercially available in the U.S.A. and Europe. The Sentinel device by Boston Scientific has been widely studied over the past decade, and its safety is undisputed. However, the approved intended use labelling does not mention that its use is associated with statistically significant reductions in either stroke rate or new cerebral lesions.17
No clinical trial has shown the Sentinel to be significantly effective at preventing strokes during TAVR. The PROTECTED TAVR randomized clinical trial primary endpoint was not met.
The use of Sentinel in the trial did not reduce overall peri-procedural stroke (control 2.9%, Sentinel 2.3% p=0.30).9 Importantly, the study also showed that the median total new lesion volume in all territories of the brain was only 5% less when Sentinel was used (294.0mm³) versus no protection (309.8 mm³) in the control arm (p = 0.8076).20
Protect the Entire Brain while
Restoring the Heart
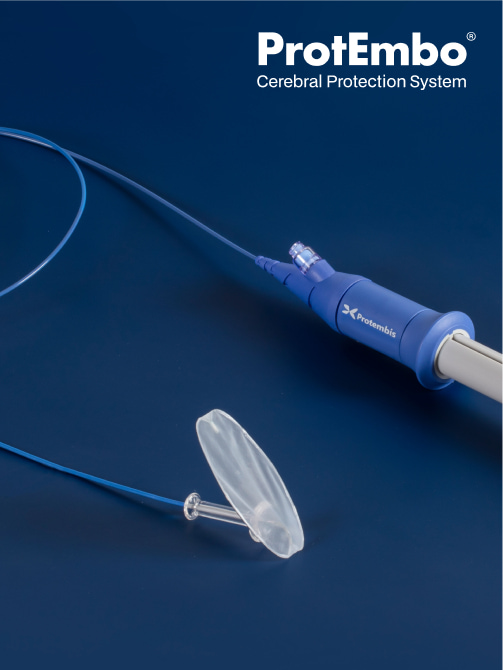
ProtEmbo CPS shields all 3 arteries that supply blood flowing to the brain, with the proximal part of the ProtEmbo device anchored in the left subclavian artery ensuring coverage of the left vertebral artery, and with the filter deployed, the rest of the frame sits in the roof of the aortic arch covering the ostia of the left common carotid and brachiocephalic arteries. As a result, the ProtEmbo CPS filters all of the blood flowing to the brain.
It has a filter with pores of 60μm designed to filter and deflect embolic material which is liberated during TAVR away from the brain.
- Bench and animal studies have optimized the pore size to allow unimpeded blood flow without increasing pressure gradients or the risk of haemolysis or thrombogenesis.
- ProtEmbo CPS pore size is approximately 60% smaller versus the pore size of the Sentinel CPS filters
A covalently bonded heparin coating on the ProtEmbo filter shield avoids the risk of additional thrombus formation.
Key features of the ProtEmbo design:
- Minimal disruption to TAVR workflow through intuitive placement in < 5 min.19
- Delivery via a 6F left radial access to reduce access site complications and alleviates catheter interaction with the TAVR device.
- Anchoring in the left subclavian artery to add stability when TAVR systems are crossing the arch.
- The frame and filter material design is ultra-low profile reducing the risk of interaction during the TAVR procedure.
- Compatible with any valve type deployed via femoral access.
How the ProtEmbo works
Promising Clinical Results
The ProtEmbo has shown promising results in clinical studies to date. Early clinical evidence suggests efficacy of entire brain protection.18
Clinical results in detail
Independent Core Lab analysis demonstrated that use of ProtEmbo led to a reduction of large new lesions when compared to historical controls.
- Majority of patients (94.1%) are free from large clinically meaningful lesions greater than 350mm³.
- Brain lesion burden overall is substantially reduced.
In the published European multicenter clinical PROTEMBO C trial with 60 patients, the ProtEmbo device met both safety and technical success primary endpoints:19
- ProtEmbo demonstrated robust safety with 30-day MACCE rate notably lower than the performance goal (p ≤ 0.0001).
- Technical procedural success rate statistically significantly greater than predefined performance goals (p ≤ 0.0002).
Caution
Publications
- Lansky et al., Clinical significance of diffusion-weighted brain MRI lesions after TAVR: Results of a patient-level pooled analysis, JACC, Vol. 84, No. 8, 2024
- Kapadia et al., Protection against cerebral embolism during transcatheter aortic valve replacement, JACC, 2017;69:367–377
- Campbell et al., Journal of Cerebral Blood Flow & Metabolism, 2022;32:50-58.
- Arnold et al., Embolic cerebral insults after transapical aortic valve implantation detected by magnetic resonance imaging, JACC Cardiovascular Interventions, 2010 Nov, 3 (11) 1126–1132
- Wityk et al., Diffusion- and perfusion-weighted brain magnetic resonance imaging in patients with neurologic complications after cardiac surgery, JAMA Neurology, 2001;58(4):571-576
- Woldendorp et al. Silent brain infarcts and early cognitive outcomes after transcatheter aortic valve,implantation: a systematic review and meta-analysis, European Heart Journal 2021;42:1004-1015
- Kapadia et al, Cerebral embolic protection during transcatheter aortic valve replacement, NEJM 2022;387(14):1253-1263. doi:10.1056/NEJMoa2204961
- Seeger et al., Cerebral embolic protection during transcatheter aortic valve replacement significantly reduces death and stroke compared with unprotected procedures, JACC, 2017, Vol. 10, No. 22
- Kapadia et al., Cerebral embolic protection during TAVR, NEJM, 2023;388(7):669. doi:10.1056/NEJMc2215783
- Vermeer et al., Silent brain infarcts: A systematic review, Lancet Neurology 2007; 6:611-9
- Blum et al., Memory after silent stroke: Hippocampus and infarcts both matter, Neurology, January 3, 2012;78 (1) 38-46
- Schmidt et al., White matter lesion progression, brain atrophy, and cognitive decline: The Austrian stroke prevention study, Annals of Neurology, 2005, Vol.58, Issue 4: 610-616
- Vermeer et al, Silent brain infarcts and the risk of dementia and cognitive decline, 2003:348;1215-1222
- Sigurdsson et al., Incidence of brain infarcts, cognitive change, and risk of dementia in the general population: The AGES-Reykjavik Study (Age Gene/Environment Susceptibility-Reykjavik Study), Stroke, 2017, Vol. 48, No. 9
- Maxwell, TAVI use rising in young US patients, yet another analysis shows, TCTMD online, May 6, 2024
- Sharma et al., National trends in TAVR and SAVR for patients with severe isolated aortic stenosis, JACC, 2022, Nov 22;80(21):2054-2056
- Sentinel CPS Instructions for Use, https://www.bostonscientific.com/content/dam/elabeling/ic/51698992- 01A_SENTINELCPS_IFU_EN_s.pdf
- Jagielak et al., Safety and performance of a novel cerebral embolic protection device for transcatheter aortic valve implantation: The PROTEMBO C trial, EuroIntervention 2022;18:590-597
- Fezzi et al., Final report of the PROTEMBO C trial: A prospective evaluation of a novel cerebral protection device during TAVI, EuroIntervention, 2024;20:e264-e267
- Kodali et al., Cerebral protection in transcatheter aortic valve replacement: The SENTINEL study, JAMA 2015
- Nazif et al., Randomized evaluation of TriGuard 3 cerebral embolic protection after transcatheter aortic valve replacement: REFLECT II, JACC, 2021, Vol. 14, No. 5