Protembis was founded in 2013 and is headquartered in Aachen, Germany. The company develops innovative technologies to reduce the risk of new significant brain lesions and stroke during cardiology procedures particularly in connection with transcatheter aortic valve replacement (TAVR).
Protection from Large Brain Lesions and Stroke in TAVR
TAVR procedures can cause large brain infarcts, which may lead to procedural clinical stroke and an increased risk of future stroke, cognitive decline, and dementia.
Technology behind
Protembis
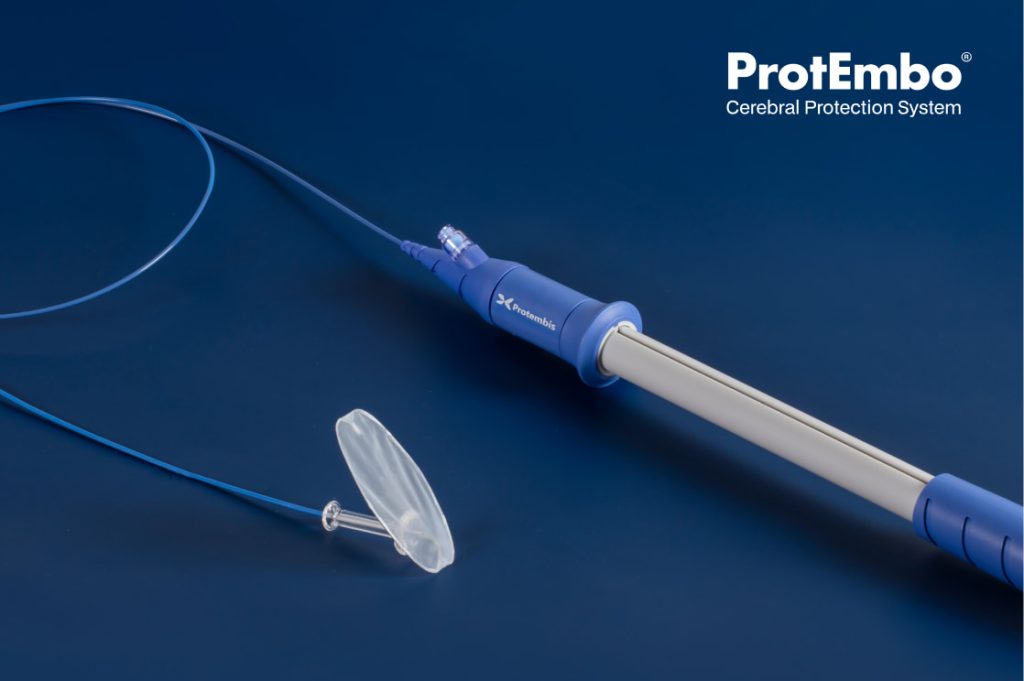
The ProtEmbo® Cerebral Protection System (CPS) brings together best-in-class next generation design properties to safely protect the whole brain from large clinically meaningful cerebral lesions.
Clinical Evidence
Focus
Clinical Studies
Ongoing
The PROTEMBO IDE Study
Prospective, multi-center, randomized, controlled trial with the goal to compare the safety and efficacy of the ProtEmbo in reducing new meaningful cerebral lesions to a hybrid control (the Sentinel CEP and No Device) in TAVR patients.
11/23/2022
The PROTEMBO C Study
The study met its predefined safety and performance endpoints while demonstrating encouraging DW-MRI data: 94% of patients treated with ProtEmbo were free of clinically meaningful large brain lesions.
02/19/2018
The PROTEMBO SF Study
Results of this study demonstrated that the ProtEmbo was safe and feasible to use. A trend to lower volumes of new cerebral lesions compared to comparison subjects was observed.