At Protembis we put
patients first

Protecting the Brain
During TAVR is Essential
Your doctor may have recommended having a procedure called transcatheter aortic valve replacement (TAVR); a new aortic heart valve is placed inside your heart so that it can function more effectively.
While TAVR is designed to improve your heart function, there is also a possibility that the procedure can cause new lesions in the brain due to dislodgment of debris (emboli). In patients with severe aortic valve disease, the risk of having a stroke within 30 days after a TAVR procedure has been reported to be 2.5 to 4.5%.

TAVR procedures can often cause silent large brain infarcts, posing risks of stroke and dementia, and potentially impacting long-term cognitive health.
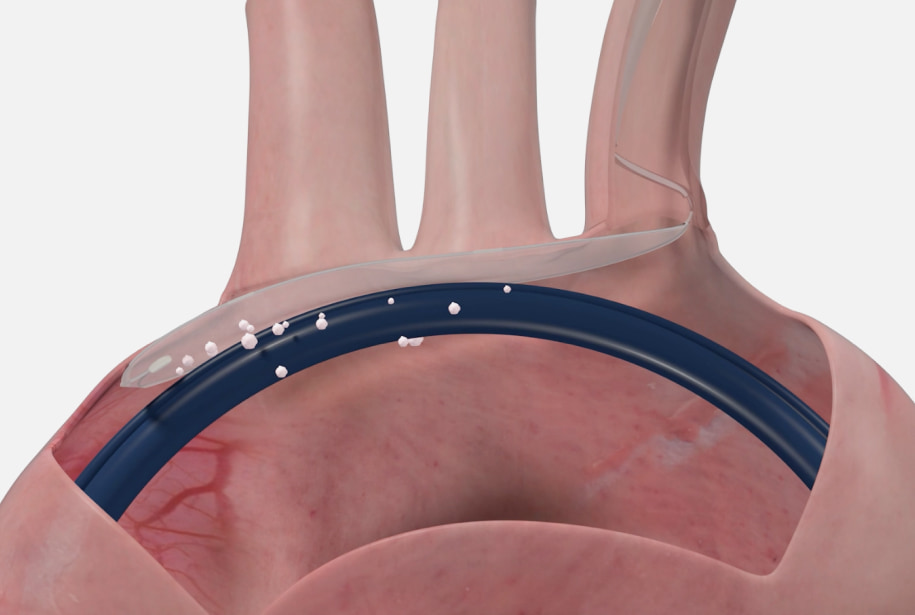
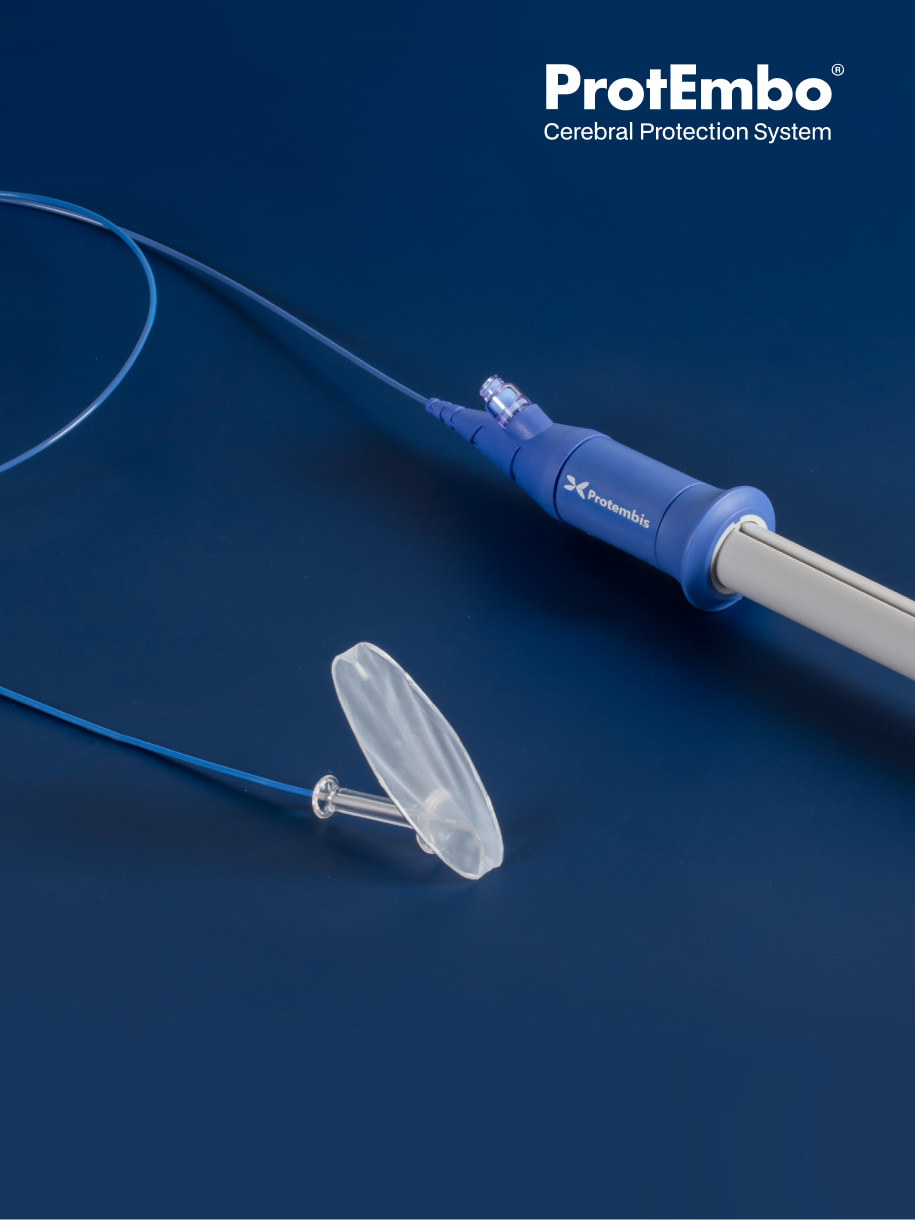
What is the ProtEmbo®
Cerebral Protection
System?
The ProtEmbo System is a new and unique device designed to protect the entire brain safely during heart procedures. Using the latest technology, the ProtEmbo is a very fine filter in the form of a shield that is designed to allow unimpeded blood flow to the brain while deflecting emboli that are released from the diseased aortic valve during the TAVR procedure.
Putting Safety First –
The PROTEMBO IDE
Trial
The PROTEMBO IDE Trial has been approved by the U.S. FDA and regulatory bodies in Europe to investigate whether using the ProtEmbo is safe and effective in helping to reduce brain injury from emboli released during the TAVR procedure.
The PROTEMBO Trial is currently enrolling patients at various clinical sites in the U.S.A., Germany and Poland.
If TAVR has been recommended to you, consult your physician to understand if you could be a candidate to participate in the trial for the ProtEmbo Cerebral Protection System during TAVR.