Putting patient safety first
The PROTEMBO IDE Study
The largest prospective, multicenter, randomized, controlled trial using DW-MRI with the goal to compare the safety and efficacy of the ProtEmbo to a hybrid control (the Sentinel and No Device) in subjects with severe symptomatic native aortic valve stenosis indicated for TAVR procedure.
Study overview
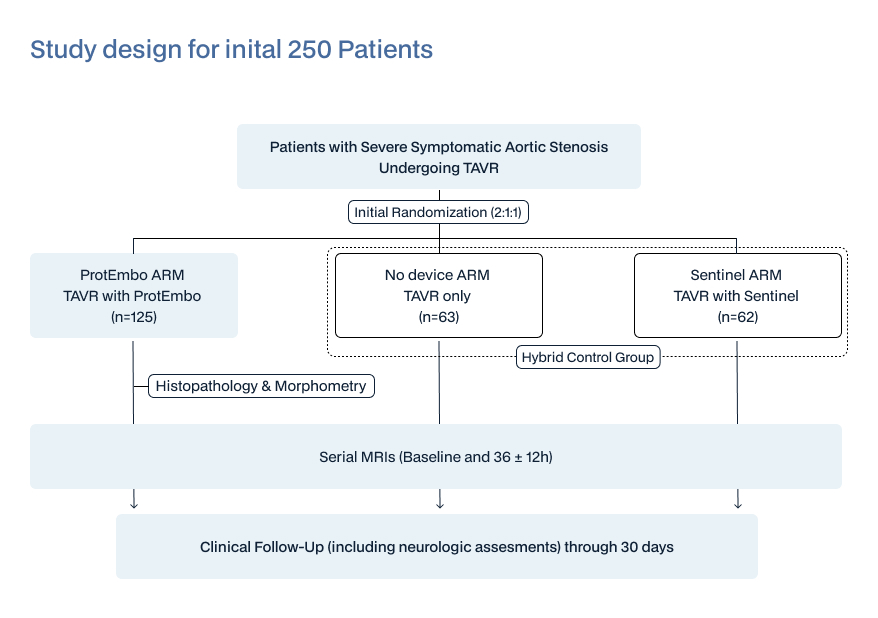
Adaptive study design with group sequential boundaries to assess outcomes at sequential interim analyses; First interim analysis at 250 patients with decision on continuation of trial based on predefined criteria of success or futility (by independent DSMB). Thereafter, further interim analyses at intervals of 50 additional patients up to a maximum of 500 patients.
Safety
Major adverse cardiac and cerebrovascular events (MACCE) at 30 days.
Efficacy
Total new lesion volume (TNLV) in the brain assessed by diffusion weighted magnetic resonance imaging (DW-MRI) at 36 ±12 hours.
Prospective, multi-centre, randomized, controlled, clinical safety and efficacy investigation
- 3 Arms: ProtEmbo, Sentinel, and No Device (i.e., no cerebral protection)
- Subjects initially randomized 2:1:1 to ProtEmbo/Sentinel/No Device
- Adaptive design with prespecified group sequential interim analyses; the first planned at 250 patients. Maximum enrolment of 500 patients.
Study Chair
Roxana Mehran
Professor, MD, FACC, FACP, FCCP, FESC, FAHA, FSCAI
Director Research & Clinical Trials at Mount Sinai School of Medicine
New York, NY, USA
Global Primary Investigators
Susheel Kodali
Assistant Professor, MD, Interventional Cardiologist
NY-Presbyterian/ Columbia University Medical Center
New York, NY, USA
Raj Makkar
Professor, MD, Director Interventional Cardiologist
Heart Institute Cedars Sinai
Los Angeles, California, USA
Stephan Haußig
MD Senior Consultant Interventional Cardiologist
Heart Center
Dresden, Germany
Previous clinical studies
11/23/2022
The PROTEMBO C study
The study met its predefined safety and performance endpoints while demonstrating encouraging DW-MRI data: 94% of patients treated with ProtEmbo were free of clinically meaningful brain lesions.
02/19/2018
The PROTEMBO SF study
Results of this study demonstrated that the ProtEmbo was safe and feasible to use. A trend to lower volumes of new cerebral lesions compared to comparison subjects was observed.